- 1Public Health Wales NHS Trust, Cardiff, United Kingdom
- 2North Wales Medical School, Health and Care Research Wales Evidence Centre, PRIME Centre, Wales, Bangor University, Bangor, United Kingdom
- 3Division of Population Medicine, Health and Care Research Wales Evidence Centre, PRIME Centre Wales, School of Medicine, Cardiff University, Cardiff, United Kingdom
Objectives: To examine the effectiveness of community diagnostic centres as a potential solution to increasing capacity and reducing pressure on secondary care in the UK.
Methods: A comprehensive search for relevant primary studies was conducted in a range of electronic sources in August 2022. Screening and critical appraisal were undertaken by two independent reviewers. There were no geographical restrictions or limits to year of publication. A narrative synthesis approach was used to analyse data and present findings.
Results: Twenty primary studies evaluating twelve individual diagnostic centres were included. Most studies were specific to cancer diagnosis and evaluated diagnostic centres located within hospitals. The evidence of effectiveness appeared mixed. There is evidence to suggest diagnostic centres can reduce various waiting times and reduce pressure on secondary care. However, cost-effectiveness may depend on whether the diagnostic centre is running at full capacity. Most included studies used weak methodologies that may be inadequate to infer effectiveness.
Conclusion: Further well-designed, quality research is needed to better understand the effectiveness and cost-effectiveness of community diagnostic centres.
Introduction
Community diagnostic centres aim to provide patients with quicker and more convenient direct access to diagnostic services and reduce pressure on hospitals, but evidence of their effectiveness and cost-effectiveness is lacking [1]. The COVID-19 pandemic directly impacted diagnostic services in the United Kingdom (UK) and globally. This, in addition to the rapid rise in demand for diagnostics that existed prior to the pandemic, has resulted in a significant backlog of patients requiring various diagnostic services and increased waiting times. Recently published data showed that in Wales, the number of patients waiting longer than the target of 8 weeks for diagnostics rose from 10.8% (7,964) in March 2020 to 41.5% (44,489) in August 2022 [2]. An Independent Review of Diagnostic Services for NHS England called for significant reform and investment in diagnostic services, and recommended the establishment of community diagnostic centres to aid in tackling the backlog and delays to diagnostic services [3]. With an emphasis on direct patient access to services from primary care, these centres can be located within hospital settings or within the community.
In England, community diagnostic centres were first launched in 2021 in a range of settings including hospitals, football stadiums, and repurposed retail outlets [1]. At present, over 90 community diagnostic centres have been opened, with plans to open up to 160 centres by 2025 [4]. In Wales, a plan to create a network of community diagnostic centres (referred to as Regional Diagnostic Hubs) was outlined by the Welsh Government in April 2022 [5]. As diagnostic services currently account for over 85% of clinical pathways within NHS England and cost over £6 billion per year [6], community diagnostic centres across a range of diagnostic services may be an effective, efficient, and cost-effective intervention for the UK health sector. These services could ensure timely diagnoses and reduced waiting times in a convenient location, ensuring people receive the treatment they need. Furthermore, community diagnostic centres could help address inequalities by providing accessible diagnostic services to people who may be less likely to engage with the healthcare system [7].
Community diagnostic centres are described within the international literature using a variety of terms and definitions. For the purposes of this review, we use the descriptor “diagnostic centres” to incorporate the range of terms used for these services. Diagnostic centres are defined here as health services aimed at improving population health outcomes by providing quicker and easily accessible diagnostic services in the community, which are accessible to primary care practitioners/services, thereby relieving pressure on secondary care services.
This rapid review aimed to examine evidence of the effectiveness and cost-effectiveness of community diagnostic centres. Preliminary work included a rapid evidence summary to identify existing systematic reviews and a rapid evidence map to highlight gaps in the evidence base [8]. This initial investigation identified a lack of recent systematic reviews and a large body of primary evidence with a broad range of outcomes in relation to diagnostic centres. Stakeholders prioritised outcomes relating to the impact of diagnostic centres on capacity and pressure on secondary care, equity in uptake or access, as well as the economic impact of these centres. This review, therefore, focussed on outcomes that were best able to demonstrate this.
Methods
Rapid reviews accelerate the process of conducting traditional systematic reviews by abbreviating or omitting various steps to produce evidence in an efficient way [9]. The methodology used for this review was developed and used by the Wales COVID-19 Evidence Centre (WCEC) during the coronavirus pandemic to inform policy decisions in Wales. They follow the methodological recommendations and minimum standards for conducting and reporting rapid reviews, including a structured protocol (not published), systematic search, screening, data extraction, critical appraisal, and evidence synthesis [10]. The structure of the review was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews [11] to allow for transparent reporting of the approaches used. Patient and public involvement (PPI) within the rapid review context is challenging, especially with the limited time frame for identifying and recruiting relevant people [12]. As part of the WCEC evidence synthesis work, members of the Public Partnership Group (PPG) provided public involvement in each review. This included participating in the stakeholder meetings, informing the review question and scope, commenting on the protocol, contributing to prioritising and defining the outcomes of interest, addressing ongoing queries from the review team, contributing to the executive summary and implications for practice, writing the lay summary, commenting on the mobilisation plan, and supporting the knowledge mobilisation and impact activities.
Literature Search
The literature search was developed during the preliminary work [8]. Resources searched included MEDLINE, EMBASE (ProQuest), Trip Medical Database (ProQuest), WHO Global, and Google Scholar. The search strategy used to search MEDLINE is available in the Supplementary File. Search concepts and keywords around diagnostic units, centres, hubs, and clinics combined free text words and descriptors when available. Searches were limited to English language publications due to time constraints. References of secondary sources identified during preliminary work were scanned for relevant primary studies and forward and backward citation tracking was conducted.
Study Selection Process
Studies included in the preliminary work (n = 50) were screened for inclusion in this rapid review using the eligibility criteria in Table 1. Screening was undertaken by five independent reviewers (AW,CO,JE,HS,AH) using the systematic review software Rayyan [13]. Disagreements were resolved by discussion amongst the review team.
Data Extraction
Data extraction and consistency checking were conducted independently by four reviewers (AW, CO, JE, HS). Information extracted included: reference details; study design; intervention/comparator; aim; data collection methods/dates; outcomes measured; study participants; setting; staffing/facilities; services provided; key findings, and any additional relevant notes (Table 2).
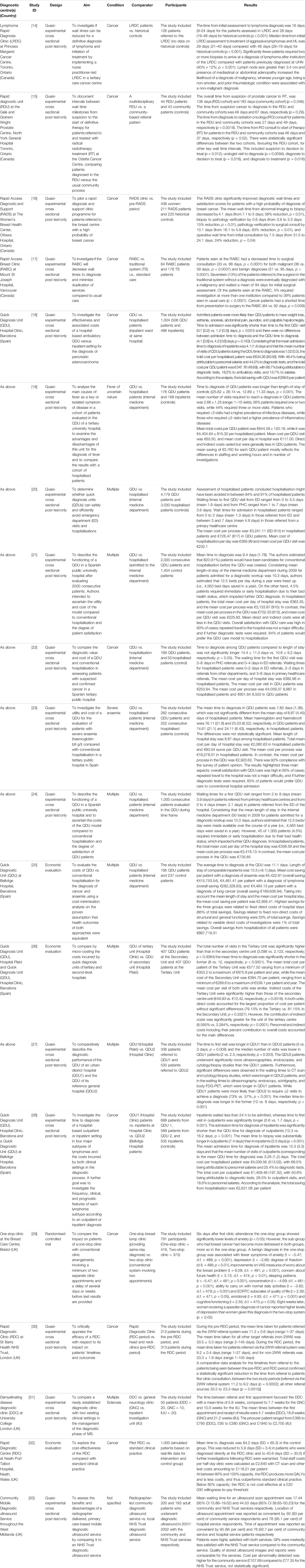
TABLE 2. Characteristics of included studies grouped by diagnostic centre. United Kingdom, August 2022.
Quality Appraisal
As the designs of included studies were often unclear, the Leatherdale algorithm was used to categorise the studies [34]. A range of study specific Joanna Briggs Institute quality appraisal checklists (randomised controlled trial [35], economic evaluation [36] and quasi-experimental [16]) were used to assess the methodological quality of included studies. Quality assessment and verification of all judgements was undertaken by four reviewers (AW, CO, JE, HS). Disagreements were resolved by discussion amongst the review team. The quality appraisal results can be seen in the Supplementary File.
Synthesis
This rapid review employed a narrative synthesis approach [24] to describe the impact of diagnostic centres on waiting times, pressures on secondary care, as well as any economic impact, and to explore relationships in the evidence found. The use of meta-analysis to synthesise quantitative findings was considered, however, due to the heterogeneity of included studies, it was not possible to undertake a valid meta-analysis. Stakeholders highlighted the importance of identifying if diagnostic centres could impact waiting times, pressures on secondary care, as well as any economic impact. As such, the outcomes identified were categorised into “impact on waiting times,” “impact on pressure” and “economic outcomes.” Where multiple studies reported outcomes on the same diagnostic centre, only the findings from the most recent study were reported to avoid the risk of double counting.
Results
A total of 20 studies was included, reporting data from 12 individual diagnostic centres. The study selection process is shown in Figure 1. Table 2 contains the characteristics of included studies grouped by the diagnostic centre they report on. Details about the characteristics of each diagnostic centre can be found in the Supplementary File. Sixteen quasi-experimental studies [14, 15, 17–23, 25–28, 30, 31, 33], three economic evaluations [29, 32, 37] and one randomised controlled trial (RCT) [38] were included. The studies were conducted in Spain (n = 11), UK (n = 5), and Canada (n = 4), and were published between 1998 and 2021. Most studies described diagnostic centres located within hospital settings (n = 19) with only one study describing a diagnostic service located within the community setting [15]. Ten studies were specific to cancer diagnoses [14, 19–21, 25, 27, 30, 31, 37, 38], six reported on the diagnosis of several health conditions [18, 22, 23, 29, 32, 33] and three studies covered a single health condition including severe anaemia (n = 1) [28], fever of uncertain nature (FUN) (n = 1) [17], and multiple sclerosis (MS) (n = 1) [26]. One study did not report a health condition of interest [15]. All studies had considerable methodological limitations and the quality of reporting was often poor. A range of performance and economic outcomes was reported and a detailed matrix of the outcomes reported by each included study can be found in the Supplementary File.
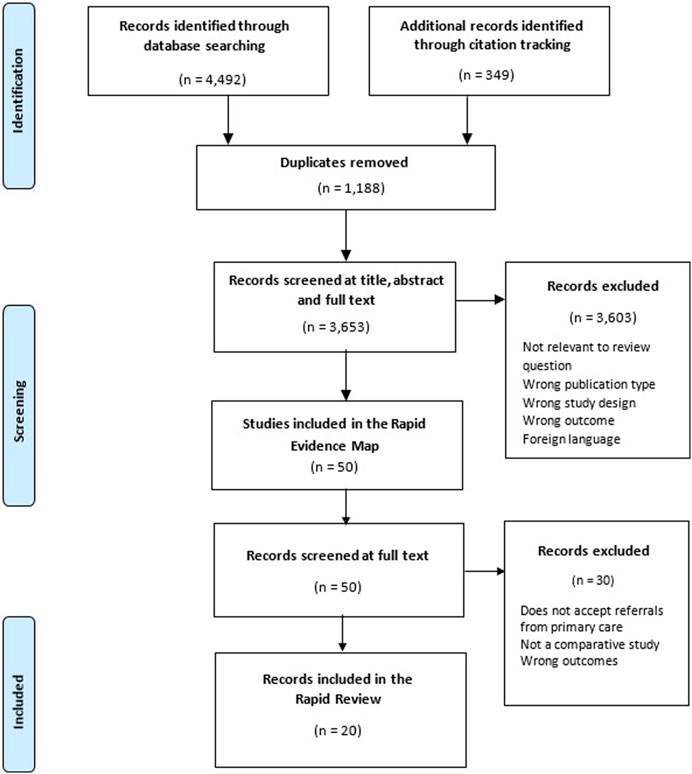
FIGURE 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagram for included studies. United Kingdom, August 2022 [11].
Impact of Diagnostic Centres on Waiting Times
Nineteen studies reported outcomes relevant to waiting times [14, 15, 17–23, 25–33, 37]. These related to different intervals of the diagnostic and treatment pathway. Some time intervals were poorly defined across the studies, to avoid misinterpretation they have been reported separately.
Time to first visit (the interval between primary care referral and first visit to the diagnostic centre) was reported for five diagnostic centres [19, 26, 27, 30, 33]. Findings were generally mixed. A reduction in time to first visit was reported in two studies for diagnostic centre patients compared to historical controls [26, 27], however the difference was only statistically significant in one study [27]. Two studies reported that the time to first visit was statistically significantly longer than the time to hospital admission for inpatients [19, 30]. When comparing two diagnostic centres, statistically significant differences were found in the median time to first visit, which was longer in the diagnostic centre of an urban district hospital than that of the diagnostic centre in a tertiary hospital [33].
Time to examination (the interval between diagnostic centre physician’s order and the examination being performed) was reported for four diagnostic centres [15, 19, 28, 33]. Study findings were generally mixed. Two studies found the mean time to examination at a diagnostic centre was shorter when compared with usual care [15, 28], while another study found a statistically significant difference in the mean time to biopsy, which was longer in the diagnostic centre than in inpatient settings [19]. When comparing two diagnostic centres, statistically significant differences were found in the time to computed tomography (CT) scan and cytology/biopsy tests, which were longer in the diagnostic centre of a tertiary hospital, while time to ultrasonography, endoscopy, scintigraphy, and positron emission tomography were longer in the diagnostic centre of an urban district hospital [33].
Time to diagnosis (the interval between the request of the decisive diagnostic procedure and the cyto/pathological diagnosis) was reported for six diagnostic centres [19, 25, 30–32, 37]. Findings were generally mixed. Three studies found the time to diagnosis to be shorter for diagnostic centre patients compared with usual care [25, 37] or historical controls [31], (two of which reported statistically significant differences [25, 31]). One study found no significant difference in the time to diagnosis between diagnostic centre and hospitalised patients [30] and one study found the time to diagnosis for diagnostic centre patients to be statistically significantly longer than for hospitalised patients [19]. When comparing different diagnostic centres a statistically significant difference was reported in the time to diagnosis, which was longer in the diagnostic centre of an urban district hospital than in the diagnostic centre of a tertiary hospital [32].
One study reported on the wait time from abnormal imaging to biopsy and from biopsy to pathology verification. Statistically significant reductions in the mean wait time for both time intervals for diagnostic centre patients compared with historical controls were identified [21].
Time to surgical consultation was reported for two diagnostic centres [14, 21]. However, these studies used different start points to measure this outcome. One study reported a statistically significant reduction in the time from pathology verification to surgical consultation for diagnostic centre patients compared to historical controls [21]. While the other reported a statistically significant decrease in the time from presentation at the clinic to surgical consultation for both malignant and benign diagnoses when attending a diagnostic centre compared to usual care [14].
The time from cancer suspicion to treatment (from suspicion by the physician or patient to radiotherapy) was reported by one study which found a statistically significant reduction in the time interval for diagnostic centre patients compared to the usual community referral process [25].
Time from consultation to therapy (from consultation with a surgeon or consultant within the diagnostic centre to treatment/surgery) was reported for five diagnostic centres [14, 21, 25, 27, 31], all focussed on the diagnosis of cancer. Two studies reported a reduction in the time from surgical consultation to surgery for diagnostic centre patients compared to historical controls [21] or usual care [14]. However, the reduction was only statistically significant in one of the studies [21]. One study reported a reduction in the time from first consultation at the diagnostic centre to the date of surgery when compared with historical controls, although this was not statistically significant [27]. Two studies reported a reduction in the time from consultation at the diagnostic centre to the start of treatment when compared to historical controls [17] or usual care [25] with one of these studies reporting the reduction to be statistically significant [31].
One study reported the time from diagnosis to radiation oncology consult was shorter for diagnostic centre patients compared to the usual community referral process and that this difference was statistically significant [25]. The same study also reported the time from diagnosis to radiation therapy and found a statistically significant reduction in time for patients attending the diagnostic centre [25].
Impact of Diagnostic Centres on Capacity and Pressure on Secondary Care
Thirteen studies reported a range of outcomes relevant to the impact of diagnostic centres on capacity and pressure on secondary care [14, 17–20, 22, 23, 26–28, 31–33].
The number of diagnostic centres patients attended to receive a diagnosis was reported by one study, which found that compared to usual care, statistically significantly more diagnostic centre patients were able to receive a diagnosis from one diagnostic centre and were not required to visit other centres for diagnostic tests [14].
The number of visits to a diagnostic centre required to obtain a diagnosis was reported for three diagnostic centres across two studies [26, 32]. Findings were inconsistent. One study reported that diagnostic centre patients required on average two visits before receiving a diagnosis compared to one to four visits, and two to five visits respectively, for the other clinical settings studied [26]. When comparing two diagnostic centres one study found that statistically significantly fewer visits were required to achieve a diagnosis at the diagnostic centre located within an urban district hospital compared to the diagnostic centre within a tertiary hospital [32].
The number of biopsies required to arrive at a definitive diagnosis was reported for one diagnostic centre [31]. Fewer patients required two or more biopsies to arrive at a diagnosis of lymphoma after the introduction of a diagnostic centre compared to usual care, and that this difference was statistically significant [31].
Referral patterns were reported for one diagnostic centre and showed statistically significant differences overtime, with more direct referrals being made to the diagnostic centre from emergency departments and less patients being hospitalised [18]. In addition, 84%–91% of hospitalised patients were found to be suitable to attend the diagnostic centre and could have avoided hospitalisation [18].
Onward referrals were reported for four diagnostic centres across three studies [19, 27, 33]. One study reported that after lymphoma diagnosis, diagnostic centre patients were statistically significantly more likely to be referred to outpatient specialist clinics and less likely to be referred to palliative care [19]. However, the authors acknowledged this was likely related to inpatients generally being older and having more aggressive lymphoma subtypes than diagnostic centre patients [19]. When comparing two diagnostic centres, those diagnosed in the diagnostic centre of the tertiary hospital were more likely to be referred to primary care centres or to the tertiary hospital’s specialised outpatient clinics compared to patients diagnosed in the diagnostic centre in an urban district hospital [33]. One study reported an increase in the number of patients in whom a definitive outcome was reached (discharged or being listed for surgery) from 33% of historical controls to 48% of diagnostic centre patients. Additionally, the number of patients requiring onward referral fell by more than half [27].
Economic Impact of Diagnostic Centres
Fourteen studies reported economic outcomes for seven diagnostic centres [15, 17–20, 22, 23, 26, 28–30, 32, 37, 38]. Of these, three were economic evaluations: one cost-minimisation analysis [25]; one cost-effectiveness study [37]; and one comparative cost analysis [32]. The other 11 quasi-experimental studies reported more generic cost data. In an attempt to highlight the more robust methodological studies (economic evaluations), these findings are reported first.
Economic Evaluations
One study used patient-level discrete-event simulation and decision analytic modelling to estimate the cost-effectiveness of a pilot diagnostic centre in its first year of operation compared with standard clinical practice in the UK [37]. During the start-up phase, the diagnostic centre saw a mean number of 2.78 patients per clinic and was more costly and more effective compared to standard clinical practice with an incremental cost-effectiveness ratio of £29,732. However, when run at near or full capacity (80% or higher, seeing a mean number of 4/5 patients/clinic), the diagnostic centre was found to outperform usual care, i.e., being less costly and more effective (incremental cost-effectiveness ratio of −£1,775/−£16,124) [37].
A comparative cost analysis using micro-costing was conducted to compare the costs incurred by two diagnostic centres located within different hospitals in Spain [32]. The mean total cost per patient in the tertiary hospital was €577.50 ± 219.60, compared to €394.70 ± 92.58 in the urban district hospital, although the mean cost per visit to both centres was similar (€182.8 ± 41.47 vs. €184.6 ± 29.41 respectively). The direct and structural costs per patient at the two centres were not significantly different. However, the indirect costs of the tertiary hospital were statistically significantly higher than those of the urban district hospital (€49.93 ± 19.90 vs. €12.42 ± 2.344 respectively). The main driver of the cost differences between the two diagnostic centres was the total number of visits and successive/first visits ratio [32].
A cost-minimisation analysis was conducted to assess the costs of the diagnostic centre approach compared with the costs of conventional hospitalisation in Spain [29]. Three groups of diagnostic centre patients (with a final diagnosis of severe anaemia, lymphoma, and lung cancer) were compared with hospitalised patients with the same diagnoses. The results showed cost savings of care delivered by the diagnostic centre compared with traditional inpatient care. The savings from hospitalisation were related to the direct costs of hospital stays (66% of savings), the non-direct costs of structural and general functioning (33% of savings) and the cost of diagnostic investigations (1% of savings). Overall savings from hospitalisation of all patients was €867,719.31 [29].
Generic Cost Data Reported in Quasi-Experimental Studies
The mean cost per diagnostic centre visit was reported for three different diagnostic centres [19, 26, 30]. Two studies reported the cost per hospital stay to be cheaper than the cost per visit to a diagnostic centre in Spain [19, 30]. A UK study found that the cost per appointment to the diagnostic centre was more expensive compared to per visit to an outpatient clinic (£395 vs. £95) but both were cheaper compared to inpatients, where the length of stay ranged from one to 5 days, with admission and testing costing £1,750 [26].
The cost of diagnostic tests per patient was reported for three diagnostic centres [15, 19, 30]. The findings suggested diagnostic tests may be cheaper in diagnostic centres situated within hospital grounds. Two studies found the total cost of diagnostic examinations per patient to be statistically significantly cheaper for diagnostic centre patients compared to hospitalised patients in Spain [19, 30]. Whereas in the UK, for patients attending the mobile diagnostic ultrasound service in the community, the cost of diagnostic tests per patient was found to be more expensive compared to the NHS Trust hospital service (£30 vs. £20.62–£27.51; respectively) [15].
The total cost per patient was reported for two diagnostic centres in Spain [19, 30]. For both centres, the total cost per patient was statistically significantly less than the total cost per hospitalised patient [19, 30]. It was shown that in the diagnostic centre 66.7% of the cost was attributable to diagnostic tests, 18.2% to ambulatory visits, and 13.7% to salaries, while the total cost per hospitalised patient included 46.4% being attributable to personnel salaries and 44.2% to diagnostic tests [30]. The average cost per process was reported for one diagnostic centre which showed the average cost to be more expensive for hospitalised patients compared to diagnostic centre patients (€3,241.11 vs. €726.47) [18].
Direct, indirect and structural costs were reported for one diagnostic centre [30]. The mean non-direct costs per patient were found to be statistically significantly less in the diagnostic centre compared to hospitalised patients and mainly corresponded to structural and general functioning costs [30]. Staffing costs were reported for two diagnostic centres, both of which identified a statistically significant reduction in staff costs compared to hospitalisation [19, 30]. Lastly, one study combined and analysed data for two diagnostic centres as a single unit and reported the total cost saving from hospitalisation to be €2,631.08 per patient [19].
Discussion
Summary of Key Findings
This review sought to examine the evidence on the effectiveness and cost-effectiveness of diagnostic centres with a particular interest in those set within the community. However, only one diagnostic service located within the community was identified, while the remaining studies covered diagnostic centres located in hospitals. Overall, the impact of diagnostic centres on waiting times appears to be mixed. The evidence suggests that diagnostic centres can reduce various waiting times, including time to surgical consultation and time from consultation to treatment. However, the evidence was mixed for other wait time outcomes including the time to first visit, time to diagnostic examination and time to diagnosis. Reductions in waiting times were reported for a number of additional intervals, although these outcomes were reported by individual studies and as such, firm conclusions cannot be made. Reducing wait times would speed up the diagnostic pathway, and could reduce the backlog of patients waiting, thereby reducing pressure in secondary care. However, although diagnostic centres may reduce diagnostic wait times, the ability to reduce the time to treatment is dependent on the capacity of the system to provide treatment. Evidence relating to the impact of diagnostic centres on capacity and pressure in secondary care in this review appears to be unclear. The evidence suggests that diagnostic centres may reduce the number of visits or the number of biopsies needed to receive a definite diagnosis, increase the number of patients reaching a clear management plan and could reduce the number of patients being referred for hospitalisation over time. However, these findings were reported by individual studies and as such firm conclusions cannot be made.
The evidence from this review suggests that diagnostic centres are cost-saving, and may be a more cost-effective resource than traditional inpatient care. However, it appears that overall cost-effectiveness may be dependent on whether the diagnostic centre is running at full capacity. Factors that could determine the costs incurred by a diagnostic centre include the diagnostic and clinical complexity of the patients, as well as the characteristics of the centre including the number of staff and contribution of staff time. Additionally, there is evidence to suggest that diagnostic centres can reduce staffing costs, costs incurred per patient, and the costs of diagnostic tests.
Findings in Relation to Previous Research
The paucity of evidence for community-based diagnostic services found in this review is in keeping with a previous literature mapping exercise and focused review [39], which identified a limited evidence base for community diagnostic services, suggesting that internationally, diagnostic centres are not commonly set up within the community. This reflects the current establishment of these sites across England, with only one in five centres opened in a community setting rather than on existing healthcare sites [7]. Whilst siting a diagnostic centre within a hospital is likely to provide greater availability to established and functioning diagnostic equipment and services, it may not be accessible to everyone and could worsen existing health inequalities. The concept of “distance decay” is recognised within the literature, with a systematic review of global north countries suggesting that those who live further away from healthcare services may have worsening healthcare outcomes [40].
However, there are potential benefits of diagnostic centres that are not dependent on their location. The literature has suggested that diagnostic centres can reduce waiting times to diagnostic tests and increase patient satisfaction [41]. A previous systematic review also found that diagnostic centres resulted in savings from hospitalisation ($2,336–$3,304) [42] supporting the findings from this review. The majority of included studies in this review compared diagnostic centre patients with a range of comparators including hospitalised patients and historical controls. These comparisons may not be appropriate considering that hospitalised patients are generally more acutely unwell and require more clinical input and longer care than those eligible to attend a diagnostic centre. This is supported by a narrative review that suggested diagnostic centres (specifically quick diagnostic centres) may be a more suitable option instead of hospitalisation for general healthy patients with suspected severe conditions [43].
Strengths and Limitations of This Rapid Review
Strengths of this rapid review include the use of a comprehensive search strategy incorporating extensive electronic database searches and review of secondary sources identified during preliminary work, quality assessment of included studies using appropriate quality appraisal tools, and the systematic approach to reporting of review findings in compliance with PRISMA guidelines.
As this is a rapid review, the methods used have been less vigorous than that of a traditional systematic review. There is therefore, the possibility that some relevant research could have been missed and there is the potential for publication bias.
Strengths and Limitations of the Available Evidence
All included studies had clear aims and objectives, and the majority had relatively large sample sizes. However, much of the evidence was derived from quasi-experimental studies with considerable methodological limitations. Key details pertaining to outcome measures or information about diagnostic centres were often lacking or poorly reported. In addition, key statistical parameters, such as confidence intervals, were not reported in some studies, making it difficult to determine the magnitude of effect of some diagnostic centres. The majority of included studies were conducted in Spain, which could limit the generalisability of our findings due to differences in healthcare systems and healthcare provision. Furthermore, many of the studies conducted in Spain reported data from the same diagnostic centres, with similar data collection periods, thereby creating the potential for double counting (see Supplementary File for details about the potential for data overlap). No studies explored equity of access to diagnostic centres and only three economic evaluations were identified, highlighting a need for further research in this area.
The diagnostic centres identified in this review varied in terms of the setting, staffing, and condition being diagnosed, further limiting the generalisability of the results. Each diagnostic centre had an individual aim, with some aiming to speed up access to diagnostic testing and overall diagnosis, and others aiming to support the patient journey by ensuring all tests can be accessed in a single day and providing follow-up support (see Supplementary File).
Implications for Policy and Practice
In light of the paucity of robust evidence, further well-designed, higher quality research is needed to better understand the effectiveness of community diagnostic centres. Research around diagnostic centres sited outside of hospital locations is particularly needed to investigate the impact on equity of access as well as the optimum location for siting these centres. In addition, further research is required to evaluate the effectiveness of diagnostic centres for conditions other than cancer, and full economic evaluations of these centres are also needed to better understand how diagnostic centres can be efficiently utilised. Policymakers would need to consider the feasibility and practicality of incorporating diagnostic centres into the healthcare sector. This would include the availability of funding for these diagnostic centres, adequate planning (including the siting of these centres), and greater inter-sector co-operation between the NHS and private sector.
Conclusion
This rapid review has highlighted possible benefits of diagnostic centres, particularly with regards to their impact on waiting times and pressure on secondary care. Although inferences around the effectiveness of community diagnostic centres cannot be made due to the paucity of evidence from diagnostic centres located outside of hospital settings, the information extracted from these studies provide valuable information into the potential benefits of establishing these centres. As diagnostic centres continue to be opened across the UK, comparative impact evaluations should be incorporated into service development plans from the onset, to assess the effectiveness of these diagnostic centres over time.
Author Contributions
AW: Search strategy, literature search, study selection, data extraction, critical appraisal, data synthesis, writing, review and editing. CO: Study selection, data extraction, critical appraisal, data synthesis, writing, review and editing. JE: Study selection, data extraction, critical appraisal, review and editing. AH: Protocol preparation, study selection, review and editing. HS: Project administration, study selection, data extraction, critical appraisal, review and editing. RL: Conceptualization, methodology, review and editing. KL, AC, and AE: Conceptualization, review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
Acknowledgments
The manuscript for this rapid review was uploaded to the preprint server medRxiv prior to journal submission. Available at: [8].
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2024.1606243/full#supplementary-material
References
1. Department of Health and Social Care. 40 Community Diagnostic Centres Launching Across England (2021). Available from: https://www.gov.uk/government/news/40-community-diagnostic-centres-launching-across-england (Accessed July 13, 2022).
2. StatsWales. Waiting Times by Month (2022). Available from: https://statswales.gov.wales/Catalogue/Health-and-Social-Care/NHS-Hospital-Waiting-Times/Diagnostic-and-Therapy-Services/waitingtimes-by-month (Accessed October 24, 2022).
3. NHS England. Diagnostic: Recovery and Renewal. Report of the Independent Review of Diagnostic Services for NHS England (2020). Available from: https://www.england.nhs.uk/wp-content/uploads/2020/11/diagnostics-recovery-and-renewal-independent-review-of-diagnostic-services-for-nhs-england-2.pdf (Accessed July 12, 2022).
4. NHS England. One Million Checks Delivered by NHS ‘one Stop Shops’. England: News (2022). Available from: https://www.england.nhs.uk/2022/06/one-million-checks-delivered-by-nhs-one-stop-shops/ (Accessed July 12, 2022).
5. Welsh Government. Our Programme for Transforming and Modernising Planned Care and Reducing Waiting Lists in Wales (2022). Available from: https://gov.wales/sites/default/files/publications/2022-04/our-programme-for-transforming--and-modernising-planned-care-and-reducing-waiting-lists-in-wales.pdf (Accessed July 13, 2022).
6. NHS. Document 3 - Community Diagnostic Hub (CDH) Draft Qualification Specification (2022). Available from: https://www.ardengemcsu.nhs.uk/media/2585/document-3-cdh-framework-specification-v111.pdf (Accessed July 12, 2022).
7. The King’s Fund. Are Community Diagnostic Centres Really Moving Care Closer to Home? (2022). Available from: https://www.kingsfund.org.uk/blog/2022/10/are-community-diagnostic-centres-really-moving-care-closer-home (Accessed November 03, 2022).
8. Wale, A, Okolie, C, Everitt, J, Hookway, A, Shaw, H, Little, K, et al. A Rapid Evidence Map of What Evidence Is Available on the Effectiveness of Community Diagnostic Centres. medRxiv [Preprint] (2022). Available from: https://doi.org/10.1101/2022.12.01.22282959 (Accessed January 25, 2023).
9. Hamel, C, Michaud, A, Thuku, M, Skidmore, B, Stevens, A, Nussbaumer-Streit, B, et al. Defining Rapid Reviews: A Systematic Scoping Review and Thematic Analysis of Definitions and Defining Characteristics of Rapid Reviews. J Clin Epidemiol (2021) 129:74–85. doi:10.1016/j.jclinepi.2020.09.041
10. Garritty, C, Gartlehner, G, Nussbaumer-Streit, B, King, VJ, Hamel, C, Kamel, C, et al. Cochrane Rapid Reviews Methods Group Offers Evidence-Informed Guidance to Conduct Rapid Reviews. J Clin Epidemiol (2021) 130:13–22. doi:10.1016/j.jclinepi.2020.10.007
11. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffman, TC, Mulrow, CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:n71. doi:10.1136/bmj.n71
12. Joseph-Williams, N, Williams, D, Gee, P, Hall, B, Strong, A, Kumar, R, et al. Involving the Public in Research Prioritisation and as Research Partners in the Wales COVID-19 Evidence Centre (2021-23): Processes, Research Priorities, Outputs, and Lessons (2023). Available from: https://www.researchsquare.com/article/rs-3624819/v1 (Accessed July 12, 2022).
13. Ouzzani, M, Hammady, H, Fedorowicz, Z, and Elmagarmid, A. Rayyan — A Web and Mobile App for Systematic Reviews. Syst Rev (2016) 5:210. doi:10.1186/s13643-016-0384-4
14. McKevitt, EC, Dingee, CK, Leung, S, Brown, CJ, Van Laeken, NY, Lee, R, et al. Reduced Time to Breast Cancer Diagnosis With Coordination of Radiological and Clinical Care. Cureus (2017) 9(12):e1919. doi:10.7759/cureus.1919
15. Pallan, M, Linnane, J, and Ramaiah, S. Evaluation of an Independent, Radiographer-Led Community Diagnostic Ultrasound Service Provided to General Practitioners. J Public Health (2005) 27(2):176–81. doi:10.1093/pubmed/fdi006
16. Tufanaru, C, Munn, Z, Aromataris, E, Campbell, J, and Hopp, L. Chapter 3: Systematic Reviews of Effectiveness. In: E Aromataris, and Z Munn, editors. JBI Manual for Evidence Synthesis. Australia: JBI (2020). Available from: https://synthesismanual.jbi.global.
17. Brito-Zerón, P, Nicolás-Ocejo, D, Jordán, A, Retamozo, S, López-Soto, A, and Bosch, X. Diagnosing Unexplained Fever: Can Quick Diagnosis Units Replace Inpatient Hospitalization? Eur J Clin Invest (2014) 44(8):707–18. doi:10.1111/eci.12287
18. Bosch, X, Jordán, A, and López-Soto, A. Quick Diagnosis Units: Avoiding Referrals From Primary Care to the ED and Hospitalizations. Am J Emerg Med (2013) 31(1):114–23. doi:10.1016/j.ajem.2012.06.013
19. Bosch, X, Sanclemente-Ansó, C, Escoda, O, Monclús, E, Franco-Vanegas, J, Moreno, P, et al. Time to Diagnosis and Associated Costs of an Outpatient vs Inpatient Setting in the Diagnosis of Lymphoma: A Retrospective Study of a Large Cohort of Major Lymphoma Subtypes in Spain. BMC cancer (2018) 18(1):276–15. doi:10.1186/s12885-018-4187-y
20. Bosch, X, Moreno, P, Ríos, M, Jordán, A, and López-Soto, A. Comparison of Quick Diagnosis Units and Conventional Hospitalization for the Diagnosis of Cancer in Spain: A Descriptive Cohort Study. A Descriptive Cohort Study Oncol (2012) 83(5):283–91. doi:10.1159/000341658
21. Arnaout, A, Smylie, J, Seely, J, Robertson, S, Knight, K, Shin, S, et al. Improving Breast Diagnostic Services With a Rapid Access Diagnostic and Support (RADS) Program. Ann Surg Oncol (2013) 20(10):3335–40. doi:10.1245/s10434-013-3120-5
22. Bosch, X, Foix, A, Jordán, A, Coca, A, and López-Soto, A. Outpatient Quick Diagnosis Units for the Evaluation of Suspected Severe Diseases: An Observational, Descriptive Study. Clinics (2011) 66(5):737–41. doi:10.1590/s1807-59322011000500005
23. Bosch, X, Jordán, A, Coca, A, and López-Soto, A. Quick Diagnosis Units Versus Hospitalization for the Diagnosis of Potentially Severe Diseases in Spain. J Hosp Med (2012) 7(1):41–7. doi:10.1002/jhm.931
24. Popay, J, Roberts, H, and Sowden, A. Guidance on the Conduct of NarrativeSynthesis in Systematic Reviews: A Product From the ESRCMethods Programme. Lancaster: Institute of HealthResearch (2006).
25. Sethukavalan, P, Zhang, L, Jethava, V, Stevens, C, Flax, S, Buckley, R, et al. Improved Wait Time Intervals for Prostate Cancer Patients in a Multidisciplinary Rapid Diagnostic Unit Compared to a Community-Based Referral Pattern. Can Urol Assoc J (2013) 7(7):244–50. doi:10.5489/cuaj.181
26. Porter, B, Keenan, E, Record, E, and Thompson, AJ. Diagnosis of MS: A Comparison of Three Different Clinical Settings. Journal (2003) 9(5):431–9. doi:10.1191/1352458503ms940oa
27. Choudhury, N, Hassen, Y, Siddiqui, J, Falzon, A, and Ghufoor, K. A Multidisciplinary Audit of Head and Neck Referrals: Considerations for Patients’ Timelines and Outcomes. Eur Arch Oto-Rhino-Laryngology (2013) 270(12):3121–6. doi:10.1007/s00405-013-2453-9
28. Bosch, X, Palacios, F, Inclán-Iríbar, G, Castañeda, M, Jordán, A, Moreno, P, et al. Quick Diagnosis Units or Conventional Hospitalisation for the Diagnostic Evaluation of Severe Anaemia: A Paradigm Shift in Public Health Systems? Eur J Intern Med (2012) 23(2):159–64. doi:10.1016/j.ejim.2011.02.013
29. Sanclemente-Ansó, C, Bosch, X, Salazar, A, Moreno, R, Capdevila, C, Rosón, B, et al. Cost-Minimization Analysis Favors Outpatient Quick Diagnosis Unit Over Hospitalization for the Diagnosis of Potentially Serious Diseases. Eur J Intern Med (2016) 30:11–7. doi:10.1016/j.ejim.2015.12.015
30. Bosch, X, Moreno, P, Guerra-García, M, Guasch, N, and López-Soto, A. What Is the Relevance of an Ambulatory Quick Diagnosis Unit or Inpatient Admission for the Diagnosis of Pancreatic Cancer? A Retrospective Study of 1004 Patients. Medicine (2020) 99(11):e19009. doi:10.1097/MD.0000000000019009
31. Nixon, S, Bezverbnaya, K, Maganti, M, Gullane, P, Reedijk, M, Kuruvilla, J, et al. Evaluation of Lymphadenopathy and Suspected Lymphoma in a Lymphoma Rapid Diagnosis Clinic. JCO Oncol Pract (2020) 16(1):e29–e36. doi:10.1200/JOP.19.00202
32. Bosch, X, Montori, E, Merino-Penas, M, Compta, Y, Ladino, A, Ramon, J, et al. A Comparative Cost Analysis Between Two Quick Diagnosis Units of Different Levels of Complexity. J Comp Effectiveness Res (2021) 10(5):381–92. doi:10.2217/cer-2020-0212
33. Montori-Palacín, E, Prieto-González, S, Carrasco-Miserachs, I, Altes-Capella, J, Compta, Y, López-Soto, A, et al. Quick Outpatient Diagnosis in Small District or General Tertiary Hospitals: A Comparative Observational Study. Medicine (2017) 96(22):e6886. doi:10.1097/MD.0000000000006886
34. Leatherdale, ST. Natural Experiment Methodology for Research: A Review of How Different Methods Can Support Real-World Research. Int J Soc Res Methodol (2019) 22(1):19–35. doi:10.1080/13645579.2018.1488449
35. Barker, TH, Stone, JC, Sears, K, Klugar, M, Tufanaru, C, Leonardi-Bee, J, et al. The Revised JBI Critical Appraisal Tool for the Assessment of Risk of Bias for Randomized Controlled Trials. JBI Evid Synth (2023) 21(3):494–506. doi:10.11124/JBIES-22-00430
36. Gomersall, JS, Jadotte, YT, Xue, Y, Lockwood, S, Riddle, D, and Preda, A. Conducting Systematic Reviews of Economic Evaluations. Int J Evid Based Healthc (2015) 13(3):170–8. doi:10.1097/XEB.0000000000000063
37. Sewell, B, Jones, M, Gray, H, Wilkes, H, Lloyd-Bennett, C, Beddow, K, et al. Rapid Cancer Diagnosis for Patients With Vague Symptoms: A Cost-Effectiveness Study. Br J Gen Pract (2020) 70(692):e186–92. doi:10.3399/bjgp20X708077
38. Harcourt, D, Ambler, N, Rumsey, N, and Cawthorn, SJ. Evaluation of a One-Stop Breast Lump Clinic: A Randomized Controlled Trial. The Breast (1998) 7(6):314–9. doi:10.1016/s0960-9776(98)90073-x
39. Chamber, D, Booth, A, Baxter, SK, Johnson, M, Dickinson, KC, and Goyder, EC. Evidence for Models of Diagnostic Service Provision in the Community: Literature Mapping Exercise and Focussed Rapid Reviews. Southampton (UK): NIHR Journals Library (2016).
40. Kelly, C, Hulme, C, Farragher, T, and Clarke, G. Are Differences in Travel Time or Distance to Healthcare for Adults in Global North Countries Associated With an Impact on Health Outcomes? A Systematic Review. BMJ open (2016) 6(11):e013059. doi:10.1136/bmjopen-2016-013059
41. Gagliardi, A, Grunfeld, E, and Evans, WK. Evaluation of Diagnostic Assessment Units in Oncology: A Systematic Review. Oncol Times (2004) 26(11):68–9. doi:10.1097/01.cot.0000292146.45952.9a
42. Gupta, S, Sukhal, S, Agarwal, R, and Das, K. Quick Diagnosis Units—An Effective Alternative to Hospitalization for Diagnostic Workup: A Systematic Review. J Hosp Med (2014) 9(1):54–9. doi:10.1002/jhm.2129
Keywords: community diagnostics, secondary care, waiting times, health policy, patient care, effectiveness, cost-effectiveness, review
Citation: Wale A, Okolie C, Everitt J, Hookway A, Shaw H, Little K, Lewis R, Cooper A and Edwards A (2024) The Effectiveness and Cost-Effectiveness of Community Diagnostic Centres: A Rapid Review. Int J Public Health 69:1606243. doi: 10.3389/ijph.2024.1606243
Received: 24 May 2023; Accepted: 08 January 2024;
Published: 23 January 2024.
Edited by:
Andrea Madarasova Geckova, University of Pavol Jozef Šafárik, SlovakiaReviewed by:
Aleksandar Višnjić, University of Niš, SerbiaDuncan Chambers, The University of Sheffield, United Kingdom
Copyright © 2024 Wale, Okolie, Everitt, Hookway, Shaw, Little, Lewis, Cooper and Edwards. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alesha Wale, alesha.wale@Wales.nhs.uk; Chukwudi Okolie, chukwudi.okolie@wales.nhs.uk; Hannah Shaw, hannah.shaw@wales.nhs.uk
 Alesha Wale
Alesha Wale Chukwudi Okolie
Chukwudi Okolie Jordan Everitt1
Jordan Everitt1 Hannah Shaw
Hannah Shaw Adrian Edwards
Adrian Edwards